With the drug menace rising in sports, athletes and coaches are always on the lookout for a new breakthrough drug, to sky rocket their muscle gain, fat loss & sports performance.
Some time back I heard about a new drug in the market called PGCL, an analogue of the drug Prostaglandin Factor 2-Alpha (PGF2-A), sold by the name of Cloprostenol Sodium, in the illegal steroid market. PGCL has never been licensed to be used in humans, and saying it has side effects, is actually an understatement of sorts.

But to understand this topic, I had to go to the very basics. It starts from EFAs (Essential Fatty Acids). However, we will just discuss the outline to understand our main topic of discussion, i.e. PGCL.
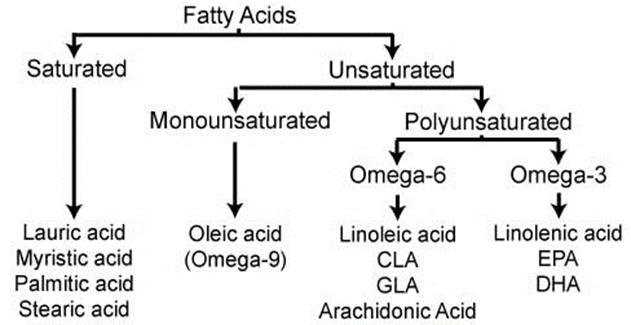
EFAs or Essential Fatty Acids, are called so, because humans cannot synthesize them in their own bodies, and need to take them from food or supplement, for maintenance of good health. They are not used as fuel for the body, but for various biological processes. Only two fatty acids are called essential: linolenic acid (omega-3 fatty acid) and linoleic acid (omega-6 fatty acid).
Our concern of discussion here starts from Omega-6 fatty acids. We will again not discuss the functions or benefits of omega-6, but what are concern is a type of Omega-6 acid called, Arachidonic Acid or AA. AA is found in membranes of the body’s cells, and is most abundant in the brain, skeletal muscles, and liver.
Further, in the body, the AA is metabolized by various enzymes, to form something called Eicosanoids, and other metabolites of eicosanoids. Eicosanoids are used in various physiological systems and pathological processes in the body such as: inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. Eicosanoids typically are not stored within cells but rather synthesized as required.
These eicosanoids are not just formed from AA, but other fatty acids also, like EPA (eicosapentaenoic acid), AdA (Adrenic acid) etc.
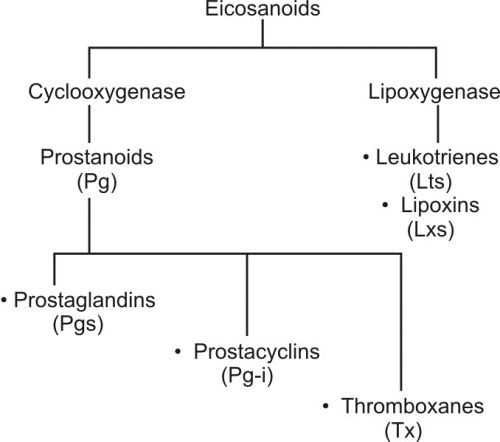
Eicosanoids have multiple subfamilies, for e.g. leukotrienes, thromboxanes, resolvins, lipoxins, prostaglandins (our topic of interest), and many more. We are interested in the prostaglandins.
Prostaglandins and their metabolites have been found in virtually every tissue in the body. The structural differences between prostaglandins account for their different biological activities. A given prostaglandin may have different and even opposite effects in different tissues in some cases. The ability of the same prostaglandin to stimulate a reaction in one tissue and inhibit the same reaction in another tissue is determined by the type of receptor to which the prostaglandin binds.
Prostaglandins (PGs) are not stored in the tissues of your body. PGs are produced in response to some physiological trigger.
Prostaglandins are classified as autocrine (effecting the same cell that produced it), as well as paracrine (effecting adjacent cells), regulators.
Prostaglandins are of different types: prostaglandin I2 (prostacyclin; PGI2), prostaglandin D2 (PGD2), prostaglandin E2 (PGE2), and prostaglandin F2a (PGF2a). All of these have various functions in the body.
But our concern of discussion again, is a specific type of prostaglandin, called prostaglandin F2a (PGF2a). It is pharmaceutically termed Carboprost, is a naturally occurring prostaglandin used in medicine to induce labour and as an abortifacient (induces abortion). When injected into the body or amniotic sac, PGF2a can either induce labour or cause an abortion depending on the concentration used. In small doses (1–4 mg/day), PGF2a acts to stimulate uterine muscle contractions, which aids in the birth process.
WHERE IT ALL STARTED
In 1930 Raphael Kurzroc and Charles Lieb reported the human uterus would either contract or relax upon instillation of fresh human semen. They characterized prostaglandin as a component of semen.
Later, Swedish physiologist Ulf von Euler in 1935 reported strong smooth-muscle stimulating activity of seminal fluid from the monkey, sheep, and goat and in extracts of the vesicular glands of male sheep. Von Euler prepared extracts of sheep vesicular glands and found the strong smooth-muscle stimulating activity. This substance was named prostaglandin.
The reason it was named prostaglandins was because, Euler thought that this substance came from prostate gland, which eventually was discovered to be produced by the seminal vesicles. Later, it was shown that many other tissues secrete prostaglandins and that they perform a variety of functions.
Research on the prostaglandins did not proceed until 1963, in contrast to the extensive research between 1935 and 1963 with progesterone and progestogens, especially for control/management of reproductive cycles of numerous mammals, especially the human. During the 1960s and 1970s the prostaglandin families were identified, characterized, and hundreds of analogs were synthesized. The first total syntheses of prostaglandin F2α and prostaglandin E2 were reported by an American organic chemist E.J. Corey in 1969. Corey later won the Nobel prize in 1990 in chemistry. In 1971, it was determined that aspirin-like drugs could inhibit the synthesis of prostaglandins.
Prostaglandins were detected in or released from lung, thymus, brain, spinal cord, kidney, iris, umbilical cord, fat, adrenals, stomach, intestines, nerves, menstrual fluid, amniotic fluid, seminal plasma, blood skeletal muscle, cardiac muscle, salivary glands, thyroid, pancreas, and uterus. Biological activity was described for cardiovascular, kidney and ureter, reproductive, gastrointestinal, respiratory, central nervous, and peripheral nervous systems.
FUNCTIONS OF PROSTAGLANDINS
Following are some of the regulatory functions of prostaglandins in various organs and systems of the body:
- Inflammation & Pain – PGs promote many aspects of the inflammatory response. They are involved in the sensation of pain associated with inflammation and vasoconstriction and/or dilation, and the development of fever. PGs, when injected directly into the hypothalamus, induce fever.

- Reproductive systems. PGs may play a role in ovulation and corpus luteum function in the ovaries and in contraction of the uterus. Excessive PG production may be involved in premature labour, endometriosis, dysmenorrhea (menstrual cramps), and other gynaecological disorders. PGs are often given to induce labour.
- Gastrointestinal tract – The stomach and intestine produce PGs. PGs are believed to inhibit gastric secretions and influence gastric motility as well as fluid absorption. Drugs such as aspirin that inhibit prostaglandin production can lead to overproduction of gastric secretion. This predisposes the person to gastric ulcers.
- Respiratory System – PGs can cause vasoconstriction as well as vasodilation of blood vessels within the lungs, depending on which PGs are being produced. PGs also cause both dilation and constriction of bronchial smooth muscle. PGs as well as other eicosanoids may play a role in asthma.
- Blood vessels – Some PGs are vasoconstrictors, others are vasodilators. The overall effect is determined by which PG is present in greater concentration.
- Kidneys – PGs are produced in the medulla of the kidneys and cause vasodilation, resulting in increased renal blood flow and increased excretion of water and electrolytes in the urine. In particular, high potassium intake has been shown to selectively increase PGF2a excretion in animals.
- Protein synthesis – PGs are known to be regulators of protein synthesis in skeletal muscle. PGE2 increases protein degradation where as PGF2a increases protein synthesis. Muscle hypertrophy is usually achieved by an increase in protein synthesis as well as a proportionately smaller increase in degradation. The simultaneous release of both PGE2 and PGF2a creates this condition.
- Adipogenesis – PGF2a directly inhibits adipogenesis (formation of fat cells). PGF2a a thermogenic, as body temperature is increased after the administration of exogenous PGF2a. Unlike other fat burners, which only decrease the size of fat cells, PGF2a actually destroys them. Fat cells die when exposed to PGF2
Current uses of PGF2a:
- Humans – PGF2a is not currently FDA approved for use in humans. Products containing PGF2a should be considered hazardous to women and must be handled with extreme care. PGF2a is readily absorbed through the skin and may result in birth defects and/or instantaneous abortion. Prostaglandins are also used for impotence in men. In such case it (PGE1) is injected directly into the penis.
- Animals – PGF2a has been tested in a wide range of animals from monkeys to horses. In most cases the side effects are increased body temperature, vomiting and diarrhoea, bronchial constriction, confusion, loss of coordination, tachycardia, and low blood pressure just to name a few. PGF2a is nontoxic with a serum half-life of only minutes. PGF2a is currently used in animal husbandry to manage breeding.
.
WHY DO BODYBUILDERS USE PGCL?
PGCL is often stacked with human growth hormone (HGH) or IGF-1 to burn body fat, in a dose of 40mcg injection, two to three times per day.

Weight loss of 1.5 to 2 pounds per day is normal, while muscle tissue becomes fuller and harder. Fat loss and vascularity is markedly improved within days, and it is far more potent than human growth hormone (HGH) in this respect. Weight losses of around 10lbs in a week are not uncommon, and unlike dieting the results tend to come very quickly and easily. At the same time, PGCL clearly acts as a diuretic which accounts for the enhanced vascularity and improved physical appearance to some extent.
In fact, even in those who run PGCL longer and lose up to 20lbs in bodyweight, the accompanying strength and muscle mass lost is relatively slight when compared to regular dieting, or even dieting with the aid of pharmaceuticals such as clenbuterol, T3 and growth hormone.
Food is removed from the gut very quickly, so subjects were able to eat a great deal of protein without feeling full. One of the most remarkable effects of PGF2A is that it mediates the major part of the anabolic effects of insulin. By using PGF2A, you can use far less insulin and get a far stronger muscle building effect.
WHY NO ONE SHOULD USE IT?

Side effects associated with PGF2a use are:
- An increase in body temperature, fall in energy, flu-like symptoms, vomiting, laboured breathing and severe abdominal pain/cramping due to dehydration.
- As PGF2a vasoconstricts the blood vessels in the lungs, the user will feel a tightening in the chest. If you’re an asthmatic, use of PGF2a could very well put you into full respiratory arrest followed by death.
- Because PGF2a increases insulinogenic effects, hypoglycaemia is a potential side-effect.
- PGF2a has a very short half-life in the body (only minutes) and most of it is metabolized in the lungs, thus making frequent injections necessary.
- PGF2A induces a very strong contraction of the Intestine and the bladder (both smooth muscles). Using a minimal dose of 40mcg (people take up to 120mcg/day), within ten minutes users will immediately have to evacuate their bowels. So, make sure you have unrestricted use of a bathroom, for at least 20 minutes. Prolonged diarrhoea for several minutes will be encountered alongside abdominal pain. At the same time, the area injected will become inflamed and painful to either touch or to contract.
- Steroids once injected survive several days in your body. PGF2A will last only several minutes though their anabolic effects will be far longer lasting (hours). It means that frequent injections are compulsory. Ideally, this would be 3-5 times/day.
- PGF2a is being used for site injection, to induce localized muscle growth, not an artificial swelling like Synthol would induce. But with continued use these gains do not seem to last. This would appear to indicate its effects are primarily related to muscle swelling rather than actual muscle growth.



